Isomers are different molecules that have the same molecular formula but are structurally different. As a result, isomers have the same number of atoms for each element, but their atomic arrangement varies. Despite having the same molecular formula, the physical properties of each molecule can vary, especially if the functional groups associated with each molecule differ. Isomerization is the process of converting one molecule with identical atoms into another molecule with identical atoms. Depending on whether the bond energy of the configurations is comparable, this may or may not occur spontaneously.
Types of Isomers
The two main types of isomerism are structural or constitutional isomerism, which occurs when the bonds between the atoms differ, and stereoisomerism or spatial isomerism, which occurs when the bonds are the same but the relative positions of the atoms differ.
Structural Isomers
Isomers differ from one another in structural isomerism—also known as constitutional isomers, because their constituent atoms are linked in different ways and sequences. The IUPAC names for structural isomers differ.
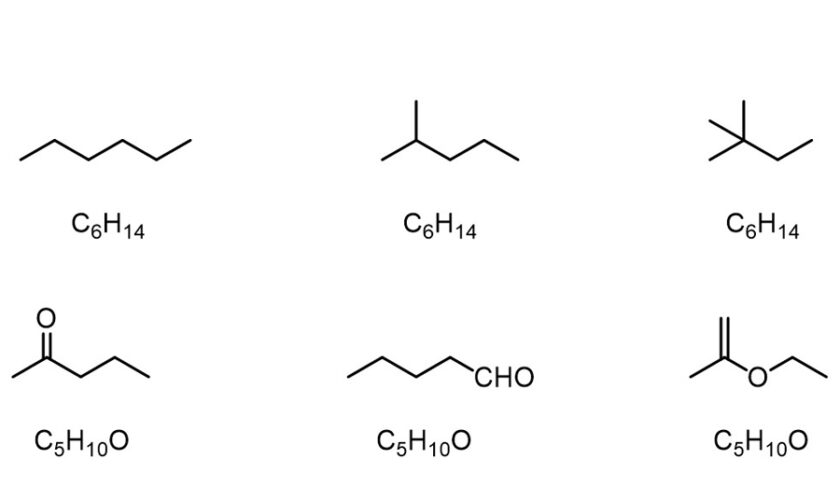
Chain isomers (e.g., hydrocarbon chains with different branching patterns); position isomers (which differ based on the positioning of a functional group on the chain); functional group isomers (in which a functional group is further divided into different functional groups); and skeletal isomers (which exhibit different carbon chains) are examples of structural isomers. A tautomer is another type of structural isomer. Tautomers spontaneously interconvert between two structural isomers, and their properties vary depending on the isoform.
Stereoisomers
Stereoisomers are isomers that have the same bond structure but differ in the geometric position of the functional groups and atoms. Enantiomers, diastereomers, and conformational isomers are examples of stereoisomers. Enantiomers are mirror images with chiral centres that cannot be superimposed. Diastereomers are not mirrored images with or without chiral centres. Different rotations occur around single bonds in conformational isomers.
Meso compounds are examples of stereoisomers.
Conformational Isomers (conformers)
Isomers can be classified using conformation. Enantiomers, diastereomers, and rotamers are all examples of conformers.
There are several methods for identifying stereoisomers, including cis-trans and E/Z.
Examples of Isomers
A few examples of isomers are as follows:
-
Methoxyethane and Propanol –
C3H8O is a chemical structure that exists as several propanol isomers, as well as the isomer methoxy ethane.
-
Methylacetylene and Allene –
C3H4 isomers include methyl acetylene and allene, which differ based on the type of bonding exhibited by the molecules.
-
Fulminate and Cyanate –
Isomers of CNO include fulminate and cyanate.
-
Glucose and Fructose –
C6H12O6 isomers include glucose and fructose, which differ based on the position of a double-bonded O atom.
- Meso Compounds
Importance of Isomers
Since enzymes prefer one isomer over another, isomers are especially important in nutrition and medicine, and enantiomers are of particular concern because they may have distinct biological activities. Many preparative procedures result in an equal mixture of both enantiomeric forms. In some cases, chiral stationary phases are used to separate the enantiomers. They can also be separated by forming diastereomeric salts. Enantioselective synthesis has also been developed in other cases.
As an example of an inorganic compound, cisplatin is an important drug used in cancer chemotherapy, whereas the trans isomer (transplatin) has no pharmacological activity.